💊New Chapter 11 Bankruptcy Filing - Sienna Pharmaceuticals Inc. ($SNNA)💊
Sienna Pharmaceuticals Inc.
September 16, 2019
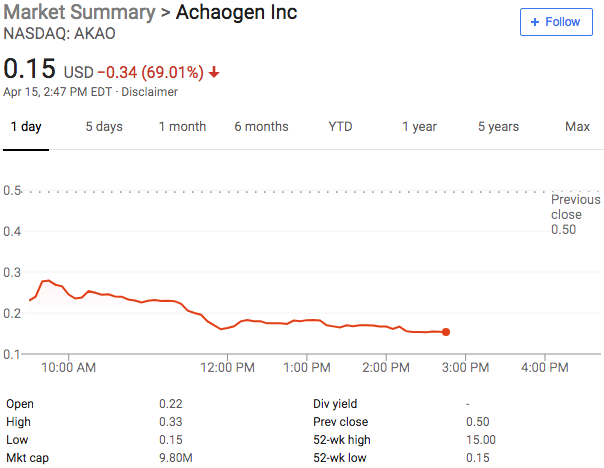
If you’re tired of distressed retail and oil & gas companies, the good news is that the biopharma space has been mixing things up. Sienna Pharmaceuticals ($SNNA), a California-based clinical-stage biopharma and medical device company filed for bankruptcy in the District of Delaware. It develops multiple products aimed at chronic inflammatory skin diseases (e.g., psoriasis) and aesthetic conditions (e.g., unwanted hair and acne). The company is at the stage that those in the tech world would designate “pre-revenue.”
And that is precisely the problem. Much like distressed oil and gas companies, distressed biopharma companies are capital intensive (a $184.1mm accumulated deficit) and tend to succumb to the weight of their long-duration development cycle. In this case, the company “has relied on equity issuances, debt offerings, and term loans” to fund development and operations. It has also leveraged its equity as a currency, engaging in strategic acquisitions that enhance its product portfolio; it, for instance, entered into a share purchase agreement in late ‘16 with Creabilis plc. This added one more product that, at this juncture, the company cannot advance due to liquidity issues. Womp womp.
The company has, over the course of time, been indebted to its pre-petition secured lender, Silicon Valley Bank, in the range of $10-30mm. On September 15th, for instance, the company owed SVB over $30mm. In exchange, however, for the use consensual use of cash collateral, the company made a $21.3mm payment to SVB on September 16th, the day before the bankruptcy filing. That’s what you call leverage, folks. SVB’s loan is secured by a laundry list of debtor assets though it is technically not secured by the company’s extensive trove of intellectual property (~250 patents). That IP, however, is subject to what’s called a “negative pledge,” a provision that prevents the company from pledging the IP on account of the fact that SVB’s security interest includes “rights to payment and proceeds from the sale, licensing, or disposition of all or any part of the Intellectual Property.” It’s a wee bit hard to enforce a security interest in IP if someone else has a right to the payments streams emanating therefrom (not that this company has any revenue streams, but you get the idea).
Why bankruptcy? For starters, the company is subject to a “minimum cash covenant” under its SVB facility and liquidity dipped below the minimum. Due to the company’s declining stock price, the company lost access to the equity market. Finally, the company has lingering financial commitments from the Creabilis deal. For all of these reasons, the company simply doesn’t have the liquidity needed to fund the next stages of product development which, in turn, would get the company closer to revenue generation. Chicken. Meet egg.
As is the overwhelming norm these days, the company now seeks to use the bankruptcy process to pursue a sale. As of the filing, no stalking horse purchaser is teed up but the company is “confident” that its banker, Cowen & Co. ($COWN), will locate one that will enable the company to emerge from bankruptcy as a going concern. No pressure, Cowen.
Jurisdiction: D. of Delaware (Judge TBD)
Capital Structure: $10mm secured debt (SVB)
Professionals:
Legal: Latham & Watkins LLP (Peter Gilhuly, Ted Dillman, Shawn Hansen) & Young Conaway Stargatt & Taylor LLP (Michael Nestor, Kara Hammond Coyle)
Financial Advisor: Force 10 Partners (Jeremy Rosenthal)
Investment Banker: Cowen and Company LLC (Lorie Beers)
Claims Agent: Epiq Corporate Restructuring LLC (*click on the link above for free docket access)
Other Parties in Interest:
Prepetition Lender: Silicon Valley Bank
Legal: Sheppard Mullin Richter & Hampton LLP (Ori Katz, Michael Driscoll) and Benesch Friedlander Coplan & Aronoff LLP (Jennifer Hoover, Kevin Capuzzi)
Major shareholders: ARCH Venture Partners VIII LLC, Partner Fund Management LP, FMR LLC (aka Fidelity)
Official Committee of Unsecured Creditors (Therapeutics Inc., Johnson Matthey Inc., MedPharm Ltd.)
Legal: Foley & Lardner LLP (Richard Bernard, Alissa Nann) & Potter Anderson & Corroon LLP (Christopher Samis, L. Katherine Good, D. Ryan Slaugh)
Financial Advisor: Emerald Capital Advisors (John Madden)
Update: 10/25/19 #140